Beginner Level
Welcome to the World of Fraction Finding
Beginner Level Syllabus
- What is spectroscopy?
- What is fluorescence?
- What are waves and wavelengths?
- What are nanometers?
- What are peak structures?
- Putting concepts into context
- Basics of the Fraction Finder
- Traditional Method vs Fraction Finder Method
- Challenges with typical fraction finding techniques
- What about color changes to determine fractions?
- Read our Beginner’s Guide to Fluorescence Spectroscopy
___
First, let’s define each term separately. We will provide basic meanings, technical meanings, and real-life examples.
1. What is spectroscopy?
- Basic meaning: The broad study of how light interacts with other light, surfaces, molecules, and other media
- Technical meaning: The branch of science concerned with the investigation and measurement of spectra produced when materials interact with, or emit, light
- Basic examples: Infrared (IR) cameras; Color measurements (like at Home Depot)
- Product examples: Arometrix Fraction Finder (fluorescence spectroscopic measurement device); Shimadzu IRTracer-100 (FTIR – Infrared Spectrophotometer); UV-1280 (Multipurpose UV-Visible Spectrophotometer); Horiba Duetta (Fluorescence and Absorbance Spectrometer)
- Types: Spectrophotometry, EEMS, IR, Absorption, Raman, and Fluorescence
- Root word: Spectra (plural form of spectrum) is a group of different types/wavelengths of both visible and invisible light. Basic examples include X-rays, Deep UV light, IR light, and Radio waves.
2. What is fluorescence?
- Basic meaning: Fluorescence refers to the light produced after a wavelength is exposed to it
- Technical meaning: Light radiation emitted by certain substances, usually visible light, as a result of being exposed to an external light source. This emitted light is generally of a different wavelength than the external light source.
- Basic examples: Fluorescent light bulbs, fluorescent paint, fluorescent shirts, white light LEDs
3. What are waves and wavelengths?
Now that we have defined the general foundation to this science, we are going to overview some key terms that, if you follow us on Instagram, you might have heard us mention.
A wave is a disturbance in the way in which particles move. To put this a bit more simply, light moves in waves, just as sound does; however, light’s waves are different. Light’s waves are classified as transverse waves.
Other transverse waves are ocean waves, guitar strings, and the ripple effect when you throw a rock in a lake. All of these examples have the same type of wave as light waves. Comparatively, longitudinal waves are different (i.e. sound waves).
Wavelengths are a physical aspect of a light wave. Wavelengths are the physical distance between periods of a light wave (i.e. distance between peak to peak).
If you notice on the Fraction Finder display’s Spectrum View (see below), the X-axis is wavelength.
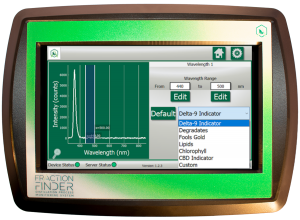
4. What are nanometers?
Nanometers are a unit of length measurement with a magnitude of 10-9.(eg. 1 meter is 0.000000001 nanometers). Wavelengths of light are generally represented in nanometers.
However, not all light is measured in nanometers.
In fact, a lot of people refer to light with eV (energy measure/photon), Hz, and even cm^(-1); the last two refer to light frequency instead of wavelength.
5. What are peak structures?
Peak structure refers to the shape of the spectral signal. Not all chemicals/molecules will have a unique Peak Structure, but they can have different, or even slightly different, peak structures which leads to them being resolvable. The Fraction Finder measures the amount of light at different wavelengths. The collection of these intensity values, with respect to the wavelengths, is what makes peak structures.
6. Putting concepts into context
An example of these concepts can be seen in our Fraction Finder Chemical Cheat Sheet. Note how we refer to each molecule’s wavelength and waveform (waveform is a colloquial term that we use to describe peak structure shape and intensity).
Fraction Finder
The first & only real-time molecule monitoring system for botanical processing.
7. Basics of the Fraction Finder
Different molecules respond to fluorescent light different. The way that a given extract or distillate responds indicates what molecules are present.
Basics of the Fraction Finder
1. Optical Sensor (attaches on equipment glassware or sight glass)
2. Digital Display (mounts to a lab pole)
3. Light-Blocking Tape (blocks ambient light from the sensor)
4. Glass Adapter (if necessary, adapts the sensor to specific equipment)
8. Traditional Method vs Fraction Finder Assisted Method
Refinement departments refine extracted plant material into more concentrated products. The potency and yield outcome of the process is based heavily on the operator’s timing in separating molecular compounds. We define the two methods for short-path distillation as the Traditional Method and the Fraction Finder Assist Method. We define the Traditional Method as using temperature, vacuum, flow, and color changes to determine fraction changes. We define the Fraction Finder Assisted Method as referencing the same process controls but in combination with a real-time molecule monitor that directly analyzes the distillation and indicates fraction changes to the operator.
9. Challenges with Typical Fraction Finding Techniques
One challenge with using the eye to determine fractions is that no two strains are exactly alike. Two adjacent runs may contain slightly different strains or impurities that may change the color. So it could be that observing a particular color on Run A indicated it was time to switch flasks. However, on Run B it may not be the case. Impurities may cause that color to come up before the fraction is ready, or perhaps after. In either case, you end up with a non-optimal result. So how do you avoid human error? The FRACTION FINDER solves that problem by showing you which fraction is being passed so you know the precise, most optimal time to switch flasks.
10. What about color changes to determine fractions?
Colorimetry is the science and technology used to detect color as perceived by humans. This technology is less subjective than the human eye, however, it relies on the same phenomenon as our technician’s eye – using color to determine fractions. This can and has worked well, however, there are other ways that are less prone to impurities and strains variety. Many variables can change color. Additionally, the FRACTION FINDER sensor can detect fractions at a much lower concentration than the human eye, while eliminating the manual task of eyeballing fraction changes.
11. Read our Beginner’s Guide to Fluorescence Spectroscopy
We recently released a guide explaining the science of fluorescence spectroscopy. You basically already read the first chapter of it in this Beginner Level, so why not just read the whole thing? Continue reading here, or move on to the Intermediate Level, where you’ll learn about relevant processes, as well as our technology’s hardware and software.
